Chemical Hazard of the Month

n-Hexane C6H14
Hexanes are significant constituents of gasoline. They are all colorless liquids, odorless when pure, with boiling points between 50 and 70°C (122 and 158°F). They are widely used as cheap, relatively safe, largely unreactive, and easily evaporated non-polar solvents.
In industry, hexanes are used in the formulation of glues for shoes, leather products, and roofing. They are also used to extract cooking oils (such as canola oil or soy oil) from seeds, for cleansing and degreasing a variety of items, and in textile manufacturing. They are commonly used in food based soybean oil extraction in the United States. (Wikipedia)
The lower explosive limit of hexane is 1.2%VOL and the upper explosive limit is 7% VOL. This means the reading of 1% LEL on a gas detector, which has been corrected for hexane, is equal to 120 ppm equivalent. From a toxic perspective, hexane has a PEL/TWA 500 ppm, a recommended TWA of 50 ppm work exposure and an IDLH of 1100 ppm. n-Hexane as an Ionization potential of 10.13eV and will be detected by a PID when using a 10.6eV lamp. If using a Rae Systems MiniRae 3000 the correction factor is 4.3 when calibrating to isobutylene.
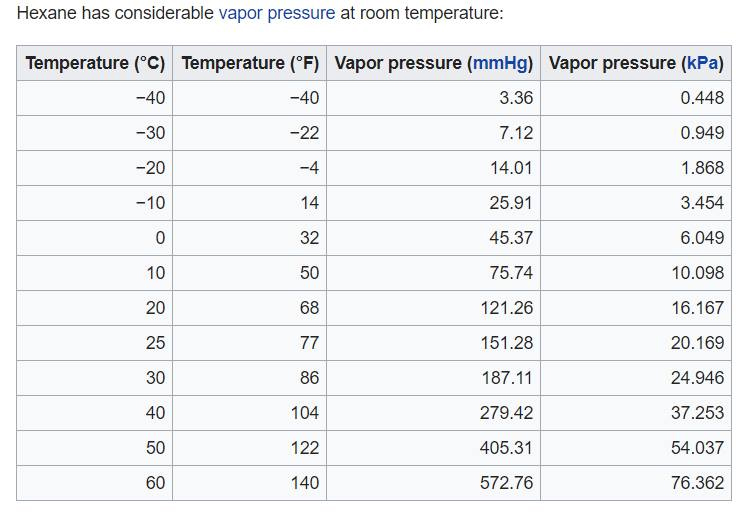
Volatility wise, hexane is considered a VOC and has a flashpoint of −14.8°F. At room temperature, a considerable amount of hexane will be in the air. See vapor pressure/temp. chart.